Capsule Summary
Evaluation of Atezolizumab Monotherapy in Metastatic NSCLC patients
The Advent of immune checkpoint inhibitors has created a paradigm shift for the treatment of numerous malignancies. Inhibitors targeting Programmed Cell Death 1 (PD- 1) receptor and its ligand PD-L1, alone or in combination with chemotherapy drugs represent the new standard of treatment for difficult to treat malignancies such as non- small cell lung carcinoma (NSCLC).
A recent study by Herbst et al. evaluated the safety and efficacy of the anti-programmed death ligand-1 (PD-L1) monoclonal antibody, Atezolizumab, in metastatic NSCLC patients. Atezolizumab is marketed as TECENTRIQ by Genentech. Following sections provide a summary of the key findings presented in the study.
Study Objective
To evaluate the safety and efficacy of Atezolizumab as the first line treatment for metastatic NSCLC, as compared to platinum based chemotherapeutic compounds, among patient groups with varying levels of PD-L1 expression.
Key methods and patient characteristics
- Randomised, open label, phase 3 trial involving patients with metastatic squamous or non-squamous NSCLC, conducted between July 21, 2015, and February 20, 2018.
- Study restricted to patients that had not received prior treatment with any chemotherapeutic agents.
- Positive PD-L1 expression on at least 1% of malignant cells or infiltrating immune cells, as assessed by SP142 immunohistochemical assay.
- Patient randomised in 1:1 ratio to receive either Atezolizumab or platinum-based chemotherapy, with a total study sample size = 572 patients. Informed consent was received from all the patients.
- Primary endpoint: overall survival evaluated according to PD-L1 expression levels.
- Safety was assessed in all the patients regardless of PD-L1 expression.
Results
No of Pts
Pts with High PDL-1 Expression
Atezolizumab
285
107 (38.6%)
Chemotherapy
287
98 (35.4%)
Adverse Events
Overall
Grade 3 or 4
Atezolizumab
90.2%
30.1%
Chemotherapy
94.7%
30.1%
Conclusion
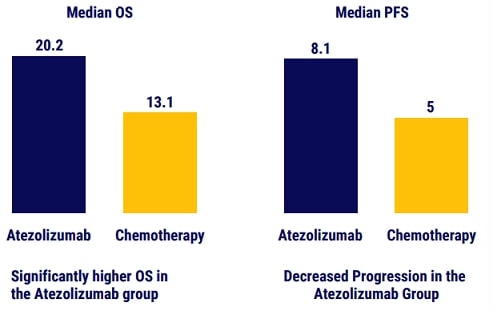
Treatment with atezolizumab monotherapy resulted in longer overall survival than platinum- based chemotherapy among patients with NSCLC with high PDL1 expression
References
The published manuscript can be accessed at – https://www.nejm.org/doi/full/10.1056/NEJMoa1917346
Information Source: Herbst et al. Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. New England Journal of Medicine, 2020 Oct 1;383(14):1328-1339. PMID: 32997907 DOI: 10.1056/NEJMoa1917346
Disclaimer
The information presented in this article is for informational and educational purposesonly and does not substitute professional medical advice and consultation with healthcare professionals.
Copyright Reserved @2021
independent Publication from Biourbexer Solutions. Please contact us at Contact@biourbexer.com for any queries.
Capsule Summary
Evaluation of Low-dose Pembrolizumab therapy in NSCLC patients
Advent of immune checkpoint inhibitors has created a paradigm shift for the treatment of numerous malignancies. Inhibitors targeting Programmed Cell Death-1 (PD1) receptor and its ligand PD-L1, alone or in combination with chemotherapy drugs represent the new standard of treatment for difficult to treat malignancies such as non-small cell lung carcinoma (NSCLC).
Although the pharmacokinetics studies using Pembrolizumab demonstrated PD-1 receptor saturation at 1 mg/kg, the clinical efficacy of Pembrolizumab in Phase III studies was evaluated at 2 mg/kg, and it is currently recommended at a dose of 200 mg every 3 weeks [1, 2]. However, biologics such as Pembrolizumab are very expensive therapeutics, and a dose reduction to achieve equivalent efficacy could be cost-effective for the patients and also reduce the overall healthcare burden.
Objective
Study objective: A study by Low et al. aimed to perform a retrospective analysis of the therapeutic benefit of low dose Pembrolizumab (100 mg) in Asian patients with advanced NSCLC [3].
Key methods and patient characteristics
- Retrospective observational study among advanced stage NSCLC patients, receiving Pembrolizumab therapy between January 2016, and March 2020 at National University Hospital, Singapore.
- 100 mg dose was routinely administered in patents lacking adequate financial resources or based on physician’s treatment preference.
- Retrospective observational study among advanced stage NSCLC patients, receiving Pembrolizumab therapy between January 2016, and March 2020 at National University Hospital, Singapore.
- Response classified as – progressive disease (PD), stable disease (SD), partial response (PR) or complete response.
- Retrospective observational study among advanced stage NSCLC patients, receiving Pembrolizumab therapy between January 2016, and March 2020 at National University Hospital, Singapore.
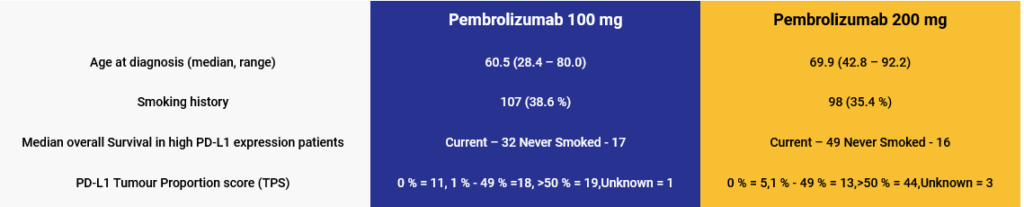
Key study characteristics:
Chemotherapy
94.7%
30.1%
Outcomes